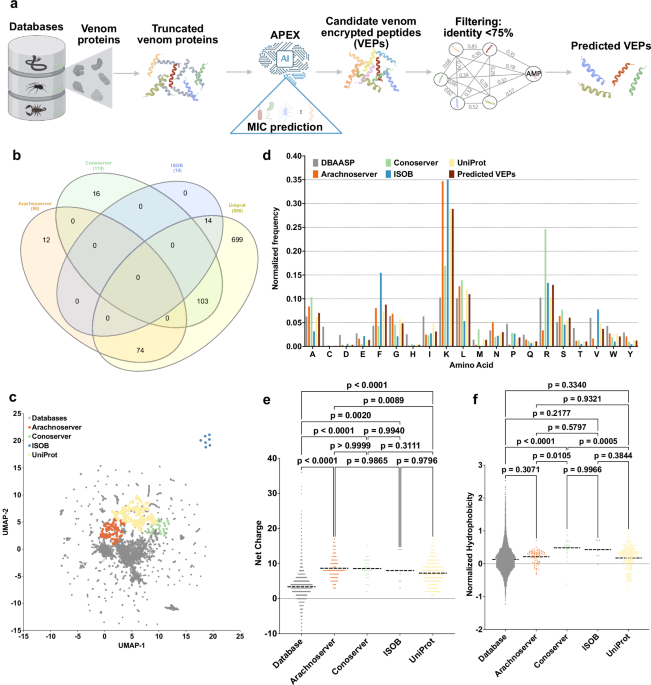
Mining venoms for antimicrobials
We sourced venom proteins from four databases: ConoServer (focusing on conopeptides, Supplementary Fig. 1)31, ArachnoServer (spider proteins, Supplementary Fig. 2)32, ISOB (indigenous snake proteins, Supplementary Fig. 3)33, and VenomZone (covering six taxa: snakes, scorpions, spiders, cone snails, sea anemones, and insects, Supplementary Fig. 4)34. The VenomZone dataset, curated from UniProtKB, was represented in our study by UniProt. Altogether, we compiled 16,123 venom proteins, which were computationally truncated (Supplementary Fig. 5) and processed to generate 40,626,260 VEPs.
To analyze differences across the databases, we performed a species overlap analysis (Fig. 1b). UniProt contained the largest number of unique species (699), reflecting its extensive coverage. Conoserver and Arachnoserver encompassed smaller unique subsets (16 and 12, respectively), while ISOB contained no unique species. These results highlight the complementary nature of these databases, emphasizing the value of integrating multiple sources to achieve comprehensive venom protein diversity.
Using APEX, a deep learning model, we predicted bacterial strain-specific MIC values for each peptide and used the median MIC as a measure of antimicrobial activity. We identified 7,379 VEPs with a median MIC ≤32 µmol L−1 (Supplementary Data 1). Further filtering criteria (see Methods: Venom-encrypted peptide selection) based on sequence similarity to known antimicrobial peptides (AMPs) yielded 386 candidates with low similarity to existing AMPs (Supplementary Table 1 and Supplementary Data 2).
To visualize sequence diversity, we compared the 386 VEPs with 19,762 known AMPs from the DBAASP database. Pairwise alignment (see Methods: Peptide sequence similarity) and uniform manifold approximation and projection (UMAP) revealed that most known AMPs clustered densely, reflecting a high sequence similarity matrix (Fig. 1c).
Most known AMPs formed a dense cluster, indicating high sequence similarity, with a minority scattered outside this cluster, representing more diverse sequences. VEPs derived from ConoServer and ArachnoServer tended to cluster closer to known AMPs, reflecting relatively higher sequence similarity. In contrast, UniProt-derived VEPs mapped farther from the AMP cluster, partially overlapping with scattered AMPs and occupying previously unexplored regions of sequence space. ISOB-derived VEPs were the most distant from known AMPs, forming isolated clusters that represent a promising source of AMP sequences (Fig. 1c).
To determine whether VEPs with low sequence similarity to known AMPs share key physicochemical characteristics, we analyzed their distribution in physicochemical feature space (Supplementary Fig. 6). While known AMPs from DBAASP clustered centrally, UniProt-derived VEPs formed three distinct clusters, Arachnoserver-derived VEPs formed two clusters, and ISOB and Conoserver each formed one cluster. UniProt cluster overlapped with ConoServer, while the ISOB-derived cluster remained entirely isolated. UniProt- and Arachnoserver-derived clusters that did not overlap with known AMPs represent unexplored regions of sequence space (Fig. 1c).
These findings suggest that our approach identifies both AMP-like peptides that differ in sequence while sharing similar physicochemical properties and entirely different AMP families that deviate in both sequence and characteristics.
Composition and physicochemical features
A comparison of amino acid composition between VEPs and DBAASP AMPs revealed distinct profiles (Fig. 1d and Supplementary Fig. 7). VEPs had lower cysteine, aspartic acid, histidine, and isoleucine, while showing higher phenylalanine, lysine, and arginine content. ISOB-derived VEPs were particularly enriched in phenylalanine, whereas Conoserver-derived VEPs displayed pronounced arginine content. Notably, Arachnoserver- and ISOB-derived VEPs were enriched in lysine.
To further understand the physicochemical properties contributing to antimicrobial activity, we benchmarked VEPs against known AMPs (Fig. 1e, f and Supplementary Fig. 8). VEPs were generally more positively charged, facilitating electrostatic interactions with the negatively charged bacterial membranes22. They alsbicity, likely driven by their increased phenylalanine and arginine content. In ISOB- and Conoserver-derived VEPs, these features enhanced amphiphilicity (Supplementary Fig. 8a), promoting secondary structure formation and membrane-associated activity.
Additionally, VEPs displayed higher isoelectric points than known AMPs (Supplementary Fig. 8b), consistent with their elevated cationic residue content. By design, the APEX model excluded peptides with high cysteine content, thereby avoiding many Conoserver-derived peptides rich in disulfide bridges. Despite their elevated phenylalanine levels, VEPs maintained comparable normalized hydrophobic moments (Supplementary Fig. 8c) and aggregation propensities (Supplementary Fig. 8d) to conventional AMPs, with amphiphilic distribution likely mitigating hydrophobic clustering.
Collectively, these results delineate the unique composition and physicochemical properties of VEPs, highlighting their potential as promising antimicrobial candidates.
Antimicrobial activity assays
Among the 58 VEPs tested, 53 (91.4%) exhibited activity against at least one pathogenic strain. Notably, all Arachnoserver-derived peptides were active, emphasizing their strong antimicrobial potential (Fig. 2a). In contrast, some UniProt-derived VEPs (from VenomZone) demonstrated limited potency: UniprotKB-2 showed no activity, while UniprotKB-6 and UniprotKB-11 were active only against Enterococcus faecium.
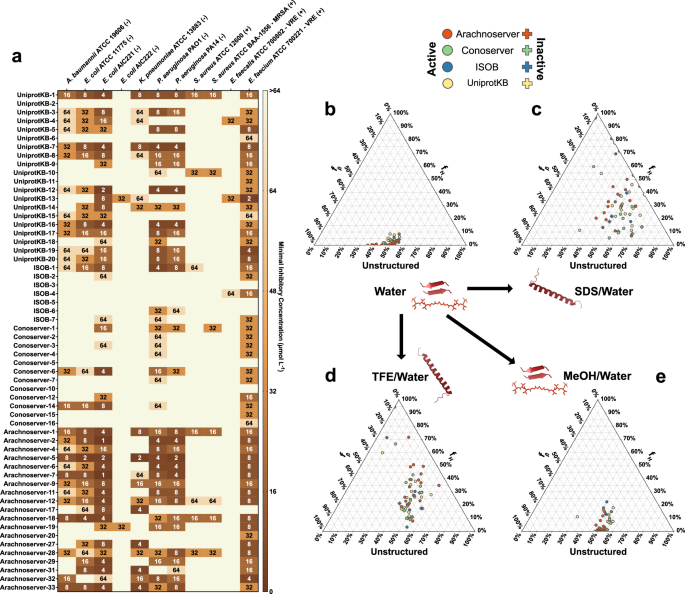
a Heatmap displaying the antimicrobial activities (μmol L-1) of active antimicrobial agents from venoms against 11 clinically relevant pathogens, including antibiotic-resistant strains (gram-negative and gram-positive bacteria as indicated as such by – and + succeeding their names). Briefly, 105 bacterial cells were incubated with serially diluted VEPs (1–64 μmol L−1) at 37 °C. Bacterial growth was assessed by measuring the optical density at 600 nm in a microplate reader one day post-treatment. The MIC values presented in the heatmap represent the mode of the replicates for each condition. b–e Ternary plots showing the percentage of secondary structure for each peptide (at 50 μmol L−1) in four different solvents: b water, c Sodium dodecyl sulfate (SDS, 10 mmol L−1) in water, d 60% trifluoroethanol (TFE) in water, and e 50% methanol (MeOH) in water. Secondary structure fractions were calculated using the BeStSel server39. Circles indicate active VEPs, while crosses represent inactive peptides.
The inactive or minimal activity of UniProtKB-2, -6, and -11 was associated with lower hydrophobicity and net charge, underscoring the important role of these parameters in facilitating membrane interactions. Conversely, ISOB-derived VEPs with enhanced normalized hydrophobicity exhibited improved antimicrobial performance. Among Conoserver-derived VEPs, an intermediate balance of hydrophobicity and net charge appeared to be optimal for activity. In Arachnoserver-derived VEPs, where all candidates were active, efficacy seemed to be driven by sequence-specific features rather than general physicochemical properties.
These findings underscore the importance of physicochemical characteristics, such as charge and hydrophobicity, in effective bacterial membrane disruption while also highlighting the significant role of sequence-specific factors in determining antimicrobial efficacy.
Secondary structure studies
The secondary structure of short peptides is inherently dynamic, often transitioning between disordered and ordered conformations depending on the surrounding environment, particularly at hydrophobic/hydrophilic interfaces. These structural transitions are critical for defining the biological functions of peptides, including their antimicrobial activity.
To investigate the structural behavior of the synthesized VEPs, we performed circular dichroism (CD) spectroscopy in diverse environments: water, sodium dodecyl sulfate (SDS)/water (10 mmol L−1), methanol (MeOH)/water (1:1, v:v), and trifluoroethanol (TFE)/water (3:2, v:v). Each medium was chosen to simulate specific physicochemical conditions relevant to peptide behavior. SDS micelles mimic biological lipid bilayers, offering a membrane-like environment conducive to evaluating interactions with bacterial membranes35. The TFE/water mixture is a known helical-inducer that promotes intramolecular hydrogen bonding by dehydrating peptide backbone amide groups, thereby favoring α-helical conformations36,37. Conversely, the MeOH/water mixture promotes interchain hydrogen bonding, stabilizing β-like structures, while hydrophobic side chains cluster to minimize contact with water, enhancing β-like conformations38.
CD spectra were recorded for all VEPs at 50 µmol L−1 over a wavelength range of 260 to 190 nm (Supplementary Fig. 9a–d). The beta structure selection (BeStSel) algorithm was employed to deconvolute the spectra and quantify the secondary structure content39 (Fig. 2b–e). As expected for short peptides (2b and Supplementary Fig. 9a, e), though with a slight propensity toward β-like conformations (fβ 9e). A similar trend was observed in the β-inducing medium (MeOH/water), where the β-content modestly increased (Fig. 2e and Supplementary Fig. 9d, e).
In contrast, VEPs exhibited a pronounced structural transition in SDS micelles (Fig. 2c and Supplementary Fig. 9c, e) and TFE/water mixture (3:2, v:v; Fig. 2d and Supplementary Fig. 9b, e), adopting α-helical conformations. This shift from disordered to α-helical structures highlights their responsiveness to membrane-mimicking environments and helical-inducing media, consistent with typical behavior observed for antimicrobial peptides6,40,41.
Interestingly, this behavior distinguishes VEPs from other classes of encrypted peptides, including those predicted by earlier proteome mining using APEX22, which predominantly adopted unstructured or β-like conformations, even in membrane-like or helical-inducing environments. Similarly, small open reading frame-encoded peptides (SEPs) and bacterial proteome-derived encrypted peptides42,43 showed limited helical propensity under comparable conditions. Instead, VEPs exhibited a structural response more akin to archaeasins, which also demonstrated a clear transition to α-helical conformations in helical-inducing media and upon interacting with lipid bilayers30. These findings suggest that VEPs may be uniquely suited for membrane-associated functions, likely contributing to their observed antimicrobial efficacy.
Mechanism of action studies
To investigate whether VEPs exert their activity via membrane-related mechanisms, we evaluated their effects on bacterial outer and cytoplasmic membranes using fluorescence assays. We used 1-(N-phenylamino)-naphthalene (NPN) assays to assess bacterial outer membrane permeabilization (Fig. 3a). Among the peptides tested, 23 VEPs effectively permeabilized the outer membrane. Notably, Arachnoserver-18, derived from the protein M-oxotoxin-Ot2d of the spider Oxyopes takobius; ConoServer-6, derived from the protein Bt211 precursor, a widely studied conotoxin from the betuline cone (Conus betulinus); and ConoServer-7, derived from the protein Con-ins G1b precursor of Conus geographus, a cone snail known for having the most potent venom among the Conus genus44, showed superior permeabilization activity. Polymyxin B and levofloxacin were used as controls in these experiments24. Overall, VEPs demonstrated permeabilization comparable to or better than other AMPs7,45,46 or other human- or animal-derived EPs22,24.
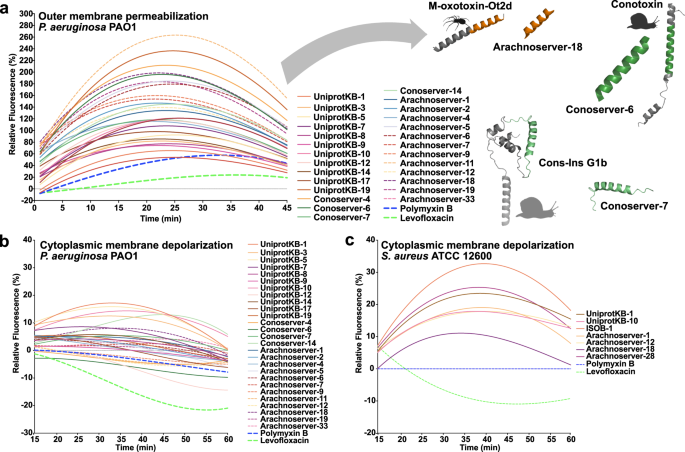
To assess whether VEPs act on bacterial membranes, all active peptides against P. aeruginosa PAO1 were subjected to outer membrane permeabilization, and peptides active against P. aeruginosa PAO1 and S. aureus ATCC 12600 were tested in cytoplasmic membrane depolarization assays. The fluorescent probe 1-(N-phenylamino)naphthalene (NPN) was used to assess membrane permeabilization (a) induced by the tested VEPs in P. aeruginosa PAO1 cells. The fluorescent probe 3,3′-dipropylthiadicarbocyanine iodide (DiSC3-5) was used to evaluate membrane depolarization of b P. aeruginosa PAO1 or c S. aureus ATCC 12600 caused by VEPs. The values displayed represent the relative fluorescence of both probes, with nonlinear fitting compared to the baseline of the untreated control (buffer + bacteria + fluorescent dye) and benchmarked against the antibiotics polymyxin B and levofloxacin. All experiments were performed in three independent replicates. The relative fluorescence values in (a–c) were calculated as the percentage difference between the sample and the untreated control using Eq. 3 (Methods). The untreated control (buffer + bacteria + fluorescent dye) served as the baseline, and polymyxin B was used as a positive control for benchmarking. The protein and peptide structures depicted in the figure were created with PyMOL Molecular Graphics System, version 3.1, Schrödinger, LLC. Panel (a) created in BioRender. De La Fuente-Nunez, C. (2025) https://BioRender.com/1bfl5da.
We next evaluated cytoplasmic membrane depolarization using 3,3′-dipropylthiadicarbocyanine iodide (DiSC3-5), a fluorophore that detects membrane potential changes. Among the 28 peptides tested against P. aeruginosa PAO1, 26 VEPs depolarized the cytoplasmic membrane more effectively than the control groups treated with polymyxin B and levofloxacin (Fig. 3b)24. However, the depolarization efficacy of VEPs was less pronounced compared to other peptide families42, such as those derived from archaeal proteomes (archaeasins)30 and SEPs42. Against the gram-positive bacterium S. aureus, VEPs exhibited slightly better depolarization activity than P. aeruginosa (Fig. 3c), though their performance remained below that of other reported peptide depolarizers23,43.
These findings suggest that VEPs primarily exert their antimicrobial effects through cytoplasmic membrane depolarization rather than outer membrane permeabilization. This mode of action aligns with that of some AMPs 45,46 and EPs24 but differs from certain computationally predicted peptides42.
In vitro cytotoxicity of VEPs
Cytotoxicity was assessed using human embryonic kidney (HEK293T) cells. Some VEPs, especially from the UniprotKB and Arachnoserver datasets, were cytotoxic at CC50 ≤ 64 µmol L−1 (Fig. 4a), mirroring their potent antimicrobial activity. To further test the toxicity of these molecules, we performed hemolysis assays by exposing the peptides to human red blood cells (Supplementary Fig. 11). In general, the peptides were not as active as against HEK293T cells, and only the most active ones presented some degree of hemolytic activity. VEPs from UniprotKB, ISOB, and Conoserver datasets were not toxic. A few Arachnoserver VEPs showed hemolytic activity and were not considered for further in vivo experimental validation. These findings underscore the importance of fine-tuning VEP properties to balance antimicrobial efficacy with reduced cytotoxicity, guiding further peptide optimization.
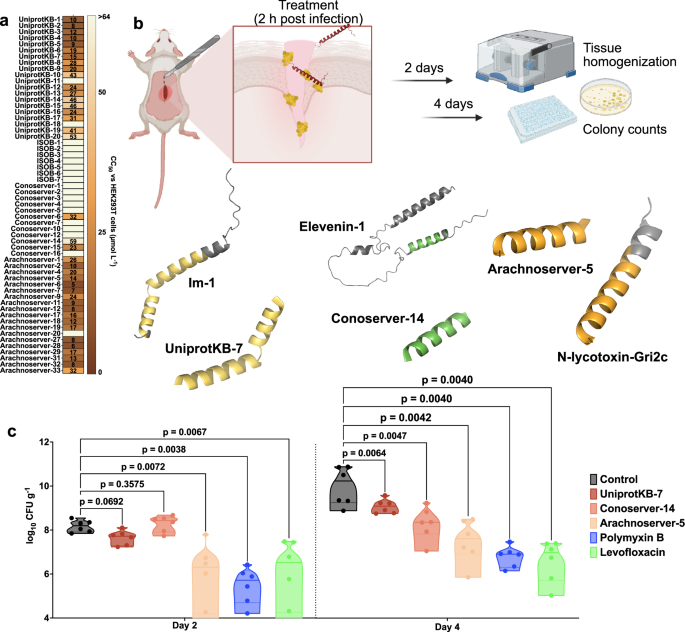
a Heatmap exhibiting the cytotoxic concentrations leading to 50% cell lysis (CC50) in human embryonic kidney (HEK293T) cells, determined by interpolating dose-response data using a nonlinear regression curve. All experiments were performed in three independent replicates. b Schematic representation of the skin abscess mouse model used to assess the anti-infective activity of VEPs (n = 6) against A. baumannii ATCC 19606. c UniprotKB-7, conoserver-14, and arachnoserver-5 were administered at their MIC in a single dose 2 h post-infection. Arachnoserver-5 inhibited the proliferation of the infection for up to 4 days after treatment compared to the untreated control group, at levels comparable to the control antibiotics, polymyxin B and levofloxacin. To determine statistical significance in (c), one-way ANOVA followed by Dunnett’s test was employed, and the respective p values are presented for each group. All groups were compared with the untreated control, and the violin plots display the median and upper and lower quartiles. Panel (b) created in BioRender. De la Fuente-Nunez, C. (2025) https://BioRender.com/ybengo5.
Anti-infective activity in preclinical animal models
To determine the in vivo efficacy of lead VEPs, we used a skin abscess mouse model infected with A. baumannii, a clinically significant pathogen (Fig. 4b). Based on their selectivity index (Supplementary Table 3), we selected the most active VEPs with low toxicity. Three VEPs demonstrated promising activity: UniProtKB-7, derived from the Im-1 toxin of the scorpion Isometrus maculatus; ConoServer-14, derived from the Elevenin-Vc1 protein of the cone snail Conus quercinus; and Arachnoserver-5, derived from the M-lycotoxin-Gri2c protein of the wolf spider Geolycosa riograndae.
A single topical dose of each VEP at its MIC significantly reduced bacterial counts 2 days post-infection. Arachnoserver-5 achieved a two-log reduction in bacterial load, comparable to the activity of polymyxin B and levofloxacin controls. Four days post-infection, all three VEPs continued to suppress bacterial growth, with Arachnoserver-5 producing a three-log reduction relative to untreated controls (Fig. 4c). Importantly, no significant changes in body weight were observed in treated animals, indicating minimal toxicity under these conditions (Supplementary Fig. 12).







